CATT Study Update 14: One-Year Study Results Show Equivalency Between Avastin and Lucentis
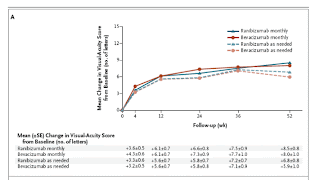 |
| Graph 1 |
 |
| Table 2 |
(By clicking on the table, an enlarged version will become visible.)
Another important observation was made concerning adverse effects. The CATT Research Group noted that "Clinical trials of intravenous Avastin in patients with cancer have identified associations with arteriothrombotic events, venous thrombotic events, gastrointestinal perforation and hemorrhage, wound-healing complications, and hypertension. With a limited statistical power to detect important adverse events, we found no significant differences between the two drugs in rates of death, arteriothrombotic events, or venous thrombotic events, findings that are consistent with the results of a study of Medicare claims involving more than 145,000 treated patients.However, in our study, the rate of serious systemic adverse events, primarily hospitalizations, was higher among Avastin-treated patients than among Lucentis-treated patients (24.1% vs. 19.0%, P=0.04). The excess numbers of these events were distributed over many different types of conditions, most of which were not identified in cancer trials involving patients who were receiving intravenous doses of Avastin that were 500 times those used in intravitreal injections. We also did not observe increased rates of adverse events with increased exposure to the study drugs; rates were higher for the two drugs when given as needed than when given monthly. The difference in rates may be attributable to chance, imbalances in baseline health status that were not included in the medical history or multivariate models, or a true difference in risk. Resolving this issue will require many more patients than were available for this study. Results from the second year of this study and from other comparative trials will provide more information regarding the relative risks of serious adverse events."
Dr. Philip Rosenfeld (the father of Avastin) provided an editorial to accompany the CATT Study results in the NEJM. Here is the full text of his editorial:
Editorial
Bevacizumab versus Ranibizumab - The Verdict
Philip J. Rosenfeld, M.D., Ph.D.
April 28, 2011 (10.1056/NEJMe1103334)
For 5 years, patients and clinicians have wrestled with the choice between two drugs for the treatment of neovascular age-related macular degeneration (AMD), a common cause of irreversible blindness among the elderly worldwide. Vision loss results from the abnormal growth and leakage of blood vessels in the macula, a specialized portion of the retina responsible for the best visual acuity. Without this macular vision, patients become legally blind. Vascular endothelial growth factor (VEGF), the cytokine primarily responsible for blood-vessel growth, is inhibited when anti-VEGF drugs are injected repeatedly into the eye, and blindness is prevented in most patients. The majority of treated patients go on to have some improvement in vision.
The two anti-VEGF drugs most commonly used are bevacizumab (Avastin) and ranibizumab (Lucentis), both developed by Genentech. Bevacizumab, a full-length humanized monoclonal antibody, has been approved by the Food and Drug Administration (FDA) for the systemic treatment of certain cancers. Ranibizumab, an antigen-binding fragment, is a smaller molecule that was specifically developed and approved to treat eye diseases and is derived from the same anti-VEGF mouse monoclonal antibody as bevacizumab. Both ranibizumab and bevacizumab bind VEGF at the same position; however, they differ in size, affinity for VEGF, speed of clearance from the eye, and cost. Ranibizumab, the FDA-approved treatment for neovascular AMD, costs approximately $2,000 per dose, whereas bevacizumab, the off-label treatment, costs approximately $50. This cost difference, along with the perceived clinical similarities between these two drugs, has led to the widespread use of bevacizumab in the absence of level I evidence.
Editors Note: According to various sources, approximately 65% of ophthalmologists in the U.S. currently use Avastin over Lucentis as their primary treatment for neovascular AMD.
In this issue of the Journal, Martin and colleagues provide such evidence in their findings from the first year of the Comparison of AMD Treatment Trials (CATT), a large, prospective, multicenter, randomized clinical trial comparing bevacizumab and ranibizumab. Despite formidable obstacles, the investigators successfully compared the two drugs and two different dosing regimens: a monthly regimen versus an as-needed regimen (i.e., drug administration only when signs of exudation are present). A monthly regimen is considered the standard for treatment. An as-needed regimen is used less frequently and relies on clinical judgment and imaging techniques to determine when to reinject the drug. The most common imaging method that is used is optical coherence tomography (OCT), a noninvasive technique that identifies fluid leakage from blood vessels. This VEGF-mediated exudate resolves after the injection of ranibizumab or bevacizumab. An OCT-guided as-needed regimen has been shown to result in improved visual acuity, but CATT is the first prospective approach to directly compare a monthly regimen with an as-needed regimen.
Martin et al. found that the monthly use of either bevacizumab or ranibizumab results in the same visual acuity outcome. This finding holds true for the mean visual acuity and the proportion of patients who gain 15 letters (which represents a doubling of the visual acuity), lose 15 letters, or remain stable. Critics will argue that the OCT outcomes suggest differences between these two drugs. Although the OCT retinal thickness measurements favor ranibizumab, this difference is not reflected in any of the visual-acuity or angiographic outcomes. Whether this difference is associated with changes in vision should become clear during the second year of follow-up.
In addition, Martin et al. observed equivalent visual-acuity outcomes with both the monthly and the as-needed regimens of ranibizumab. This result is particularly good news for patients. The success of the as-needed regimen in a multicenter clinical trial cannot be overstated, given the intrinsic difficulties associated with the training of investigators to agree on OCT interpretation and retreatment guidelines. Given deficiencies that were reported by the reading center, it is likely that visual acuity and anatomic outcomes would have been even better with improved investigator compliance. Other strategies to improve overall treatment outcomes might include the use of newer OCT techniques with improved image resolution to help with retreatment decisions and the use of three mandated monthly injections at the start of the study.
Although the as-needed regimen with bevacizumab appeared similar to the as-needed regimen with ranibizumab, the as-needed bevacizumab regimen compared less favorably with monthly regimens for either bevacizumab or ranibizumab. One possibility may be that bevacizumab has a less durable treatment effect in a subgroup of patients and thus more frequent administration may be required. If the frequency of administration were increased, then the outcomes in such patients should approach the outcomes observed with monthly treatments.
Although CATT addresses the question of efficacy, the study was insufficiently powered to identify differences in drug-related adverse events. Although bevacizumab persists longer than ranibizumab in the systemic circulation after an intravitreal injection, Martin et al. observed none of the expected adverse events associated with systemic anti-VEGF therapy. Although more patients receiving bevacizumab had multiple systemic serious adverse events and hospitalizations than those receiving ranibizumab, these events were not associated with organ systems typically identified with systemic anti-VEGF therapy. The results from the second year of CATT and from five other large, ongoing, multicenter comparative clinical trials in Europe should help to clarify whether these adverse events are related to intravitreal anti-VEGF therapy.
The CATT results, together with the totality of global experience, support the use of either bevacizumab or ranibizumab for the treatment of neovascular AMD. An as-needed regimen is an acceptable alternative to a monthly regimen, but strict compliance on the part of both the clinician and the patient is required. Health care providers and payers worldwide will now have to justify the cost of using ranibizumab. Regulators in certain countries will be forced to reconsider their policies that make it illegal to use drugs off-label, particularly when so many of their citizens cannot afford ranibizumab. The CATT data support the continued global use of intravitreal bevacizumab as an effective, low-cost alternative to ranibizumab.








